Berechtigte Hoffnungen in die guten Bio-Wissenschaften?
- die bösen haben SARS-CoV-2 mag sein erst gemacht?
Éspoirs justifiés dans les bonnes bio-sciences?
- les méchants il se peut l'aurait d'abord fabriqué?
Speranze giustificate nelle buone Bio-scienze?
- le cattive puó darsi l'avrebbero fabbricato?
Die Pandemie hat Menschen bereits zu rasanten Fortschritten
insbesondere bei den Impfstoffen gegen SARS-CoV-2 - angespornt.
Ähnliches wünschten wir uns 2022 auch zur besseren...
Bewältigung der Depressionen.
Unten erweisen wir hier auf Neuigkeiten
zu Biowissenschaften gegen Depressionen.
La pandémie a déjá conduit les humains à des progrés rapides
notamment pour les vaccins contre SARS-CoV-2.
Nous nous souhaitons le même pour une meilleure
maîtrise des dépressions.
En bas nous signalerons des nouvelles des
biosciences contre les dépressions.
La pandemia ha già stimolato gli umani verso rapidi progressi
soprattutto nei vaccini contro SARS-CoV-2.
Ci auguriamo progressi simili per
affrontare meglio la depressione.
Sottostante segnalazioni di novitá dalle
bioscienze contro le depressioni.
Neues / Du nouveau / Delle novitá / News
2023/1: Neues zu elektro-magnetischen Hirntherapie-
Verfahren im Schweizer Wissenschafst-Magazin "Horizonte"
aktuell und lesbar erklärt.
2023/2: Neues (aus Harvard und Iran) zur im Ansatz sehr interessanten "photobio-modulatorischen" Nasen-Licht-therapie mit wohl noch nicht ganz zulassungsfähigen "Proneurolight"-Geräten! 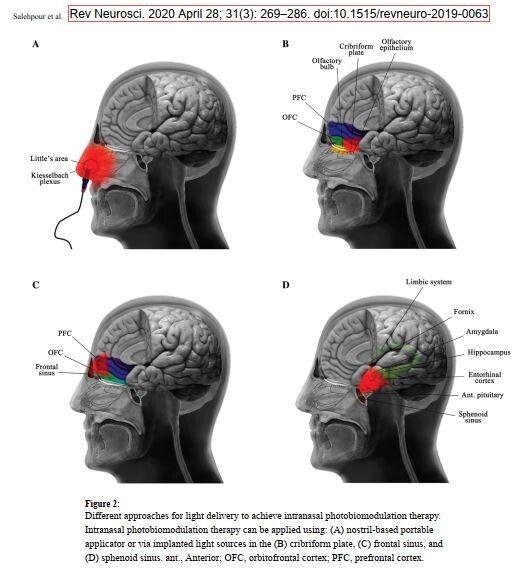
2020/1. ENERGIE-GELADEN GEGEN DEPRESSIONEN. (clic!) - Alexander Karabatsiakis, EDA-Repräsentant für Österreich.
2020/2. Biology trends in Depression 2020: THE TREMENDOUS CHALLENGE of Covid-19. - Gottfried R. S. Treviranus, EDA-Repräsentant für die Schweiz.
Biology trends in Depression 2023:
THE TREMENDOUS CHALLENGE of Covid-19.
Gottfried R. S. Treviranus, Berne
The humans are still beeing tremendously challenged by the pandemic even in some of the relatively spared high-consuming, well-
equipped societies, which revealed unsuspected voids of resilience and an escalation of disparities. The toll among frontline
(para-)medics has been appalling, shattering trust in recent sanitary policies. All this in the humans triggers strong emotional
reactions, but not necessarily depressions, which are illnesses, probably even more often grafted on already previous «affective»
temperaments, than dire consequences of noxious stress by themselves.1 The roads to depression from the pandemic obviously are
many, and those via deteriorating medical, anti-infectious, and mental care can be predicted to be as important as those from
exhausting psycho-social worry and despair. Here we focus on the possible direct tracks from Covid-19 to depression.
Only 3% of the initial published scientific output on the medical issues of the COVID-19 pandemic concerned the damages to mental
health,2 as an account from the U.S.A. mirroring the effects during the early phase in a way representing the overall population now
remarks.3 An even more minute proportion covers observations of putative mechanisms involved in the specific biological
contribution to the depressive and other loads burdening human mental health.
Here we try to catch a glimpse of first insights most directly related to biological issues of depression from Covid-19, the equally
dangerous SARS and MERS, and from what is known about the other coronavirus affecting the CNS of humans (HCovs) like the four
others causing one in three common colds.4 Researchers now zoomed in on weaknesses of human defence against Covid-19, and
notably on the natural immune responses` interferon-type1-systems either through genetical variants in 4%5 or through anti-INFg-
antibodies, a mechanism known from Mycobacteria, Staphylococcus, and Candida,6 all subverting immune cells by colonization,
which again may be central to neuropsychiatric disorders as far as related to mast cells and arteries.7
The astonishing skills in subverting bodily (and mental) functions of the COVID-19 virus, which is a member of this increasingly
infamous group of 7 human viruses, are encoded in an encapsulated huge single strand of RNA. These skills rapidly spurned theories
on usurpation of physiological, particularily immune networks8, mast cell involvement,9 cerebral auto-immunity, the origin of age-
and sex-correlations, the arterial aneurysms creation, the role of antibodies favoring receptor and clathrin-facilitated entry, the
pharmacodynamics of the few testable drugs, the peculiarities of the young either protected or not, and many more.10
How Covid-19 might enter proper CNS-tissues
Broad neurological symptoms in a quarter of Covid-19-illnesses are proportional to overall severity, comorbidity, and age, but may
also devastate children. As far as these many and variagate neuropsychiatric disturbances and lesions to the CNS are concerned, the
modes of entry of theses virus into the brain through its barriers and into its proper tissue cells are approached via tentative
models. General cell entry requires the ACE2-receptor, which again interacts with TMPrSS211 - both effectively blocked by drugs12
-and neuropilin-113, and it its followed by clathrin-mediated endocytosis – blockable by cheap and common phenothiazines14,15,16
among other useful psychotropics like lithium17 or clomipramine18 - and replication with lysosomes participating,19 one of the places
hydroxychloroquine acts.20
Some CoV can enter the neurons – were they rush to the endoplasmatic reticulum of cells21 or may persist22 - via peripheral nerves
crossing synapses or via the neural olfactory bulb above the ethmoïd cells. A review from Thessaloniki23 next to the nerval routes
and hematogenous spread mentions «nasal epithelium in general» and «lymphatic tissue». In fact air pollution particles
reaching the nose can function as vectors carrying Covid-19 too.24 While Covid-19 is assumed to be carried in the blood stream
especially of arteries and arterioles, special complex and variegate barriers made up of many other cells protect the neural tissues.
Covid-19 has been erroneously identified25 within arterial endothel (what the same institution which fallaciously smeared26 the use
by front-line doctors of hydroxy-chloroquine27 denies)28 - which would have expalined the proven replicatory invasion of an
endothelial organoid,29 particularly enlightening, as no immunocyte contribution war required. Autopsy finding instead showed their
dense perivascualr presence, and a much incrased angiogenesis and microthrombotic activity.30 That Covid-19 could colonize
macrophages, which thereafter would spread these «trojan horses» was observed,31 but not extended to brain parenchyma
invasion, which normally is not achieved by them,32 but instead by mast cells,33,34 which can be colonized by many germs or
viruses,35 and are also required for CNS-autoimmunity.36
The 2021 extension of this article also covering long-COVID-19 (PASC)
is available as conference paper of Censtupsi Perugia
in Psichiatria danubina(Zagreb).
1Gonda X, et al. Nature and Nurture: Effects of Affective Temperaments on Depressive Symptoms Are Markedly Modified by Stress Exposure. Front Psychiatry. 2020;11:599. link. 2 EppiCentre Social Science Research Unit, COVID-19: A living systematic map of the evidence. link. 3 Holman EA et al.. The unfolding COVID-19 pandemic: A probability-based, nationally representative study of mental health in the U.S.A. Sci Adv. 2020;eabd5390. link. 4 Arbour N et al.. Neuroinvasion by human respiratory coronaviruses. J Virol 2000;74:8913–21 link. 5 Zhang SY et al. COVID Team. Severe COVID-19 in the young and healthy: monogenic inborn errors of immunity? Nat Rev Immunol. 2020 Aug;20(8):455-456. link. 6 Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al.. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020 Sep 24:eabd4585. link. 7 Treviranus GRS. Psychoses by Attacks from Subverted Mast Cells: A Role for Arterial Intramural Flow Badly Steered by the Nasal Ganglia? Psychiatr Danub. 2020 Sep;32(Suppl 1):93-104. link. 8 Curran CS et al. COVID-19 Usurps Host Regulatory Networks. Front Pharmacol, 11:1278. link 9 Raymond M, Ching-A-Sue G, Van Hecke O. Mast cell stabilisers, leukotriene antagonists and antihistamines: A rapid review of the evidence for their use in COVID-19. CEBM May 18, 2020 link. 10 Zhang SY et al. COVID Team. Severe COVID-19 in the young and healthy: monogenic inborn errors of immunity?. Nat Rev Immunol. 2020;20(8):455-456. link. 11 Heurich A et al.. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014 Jan;88(2):1293-307. link.12 Hoffmann M et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16;181(2):271-280.e8. link.13 Davies J et al. Neuropilin‑1 as a new potential SARS‑CoV‑2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID‑19. Mol Med Rep. 2020 Nov;22(5):4221-4226. link. 14 Otręba M et al. Antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards RNA-viruses. A review. Eur J Pharmacol. 2020 Sep 16;887:173553. link.15 Stip E. Psychiatry and COVID-19: The Role of Chlorpromazine. Can J Psychiatry. 2020 Oct;65(10):739-740. link.16 Plaze M et al. Repositionnement de la chlorpromazine dans le traitement du COVID-19 : étude reCoVery [Repurposing of chlorpromazine in COVID-19 treatment: the reCoVery study]. Encephale. 2020 Jun;46(3S):S35-S39. French. link. 17 Sato SB et al. Wortmannin and Li+ specifically inhibit clathrin-independent endocytic internalization of bulk fluid. J Biochem. 1996 May;119(5):887-97. link. 18 Javelot H et al. Psychoactive compounds as multifactorial protection factors against COVID-19. Ir J Med Sci. 2020 Aug 18:1–2. link. 19 Amini Pouya M et al. Classification of the present pharmaceutical agents based on the possible effective mechanism on the COVID-19 infection. Daru. 2020 Jul 30:1–20. link. 20 Shukla AM et al. Chloroquine and hydroxychloroquine in the context of COVID-19. Drugs Context. 2020 Apr 28;9:2020-4-5. link. 21 Santerre M et al. Why do SARS-CoV-2 NSPs rush to the ER? J Neurol. 2020 Sep 1:1–10. link. 22 Bleau C et al. Brain invasion by mouse hepatitis virus depends on impair ment of tight junctions and Beta interferon production in brain microvascular endothelial cells. J Virol. 2015;89(19):9896–908. link. 23 Lima M et al. Unraveling the Possible Routes of SARS-COV-2 Invasion into the Central Nervous System. Curr Treat Options Neurol. 2020;22(11):37. link. 24 Setti L et al. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ Res. 2020 Sep;188:109754. link. 25 Goldsmith CS et al. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395(10238):e99. link. 26 FOR BETTER SCIENCE, 5.06.2020: forbetterscience.com/2020/06/05 27 Mehra MR et al.. Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395(10240):1820. link.28 Varga Z et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417-1418. link. 29 Monteil V et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020 May 14;181(4):905-913.e7. link. 30 Ackermann Met al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020 Jul 9;383(2):120-128. link.31 Park MD. Macrophages: a Trojan horse in COVID-19?. Nat Rev Immunol. 2020;20(6):351. link 32 Faraco G, et al. Brain perivascular macrophages: characterization and functional roles in health and disease. J Mol Med (Berl) 2017; 95:1143-52 33 Theoharides T: Mast cells: The immune gate to the brain. Life Sc 1996; 46:607-17 34 Erickson MA, Banks WA. Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol Rev. 2018;70(2):278-314. link. 35 Treviranus GRS. Psychoses by attacks from subverted mast cells: a role for arterial intramural flow badly steered by the nasal ganglia? Psychiatria Danubina, 2020 32, S2: 239–64. 36 Russi AEet al. Meningeal mast cell-T cell crosstalk regulates T cell encephalitogenicity. J Autoimmun. 2016;73:100-110. link.

